
Account in Synlett is online
April 17, 2024From protein structures to functional biomimetics
C Durukan, TN Grossmann Synlett DOI: 10.1055/a-2308-1795
Chair of Biomimetic Chemistry
Department of Chemistry and Pharmaceutical Sciences
Amsterdam Institute of Molecular and Life Sciences

For more news, visit us at Twitter:
@TomNGrossmann

From protein structures to functional biomimetics
C Durukan, TN Grossmann Synlett DOI: 10.1055/a-2308-1795
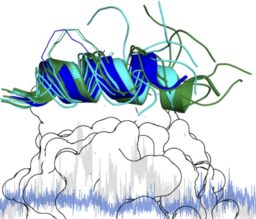
Binding dynamics of a stapled peptide targeting the transcription factor NF-Y
C Durukan, F Arbore, R Klintrot, C Bigiotti, IM Ilie, J Vreede, TN Grossmann*, S Hennig* ChemBioChem DOI: 10.1002/cbic.202400020
Great team effort and congratulations to everyone! Thanks to the MD contribution from our University of Amsterdam colleagues!

Felix successfully defended his thesis today!
Big thanks to co-promotor Dr. Joen Juirink!
Read more about Felix’ work here: J. Am. Chem. Soc. 2022, 144, 15303–15313

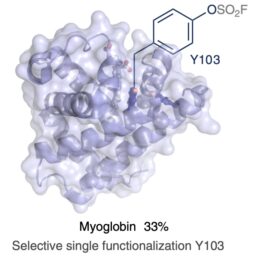
A modular flow platform for sulfur(VI) fluoride exchange ligation of small molecules, peptides and proteins
M Bernús, D Mazzarella, J Stanić, Z Zhai, A Yeste-Vázquez, O Boutureira, AFG Gargano, TN Grossmann, T Noël* Nature Synth. DOI 10.1038/s44160-023-00441-0
Congrats to the Noël group and great to be part of the team!
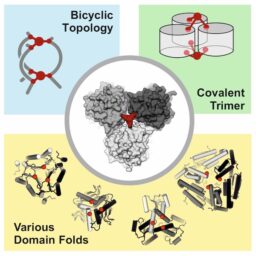
Covalent Bicyclization of Protein Complexes Yields Durable Quaternary Structures
GH Hutchins, et al. Chem. DOI: 10.1016/j.chempr.2023.10.003

Juan joins us from Łukasz Berlicki’s group at Wroclaw University.
We’re happy about the additional peptide design and synthesis expertise!


Yun joins our group with support from the Chinese Scholarship Council. Congratulations!
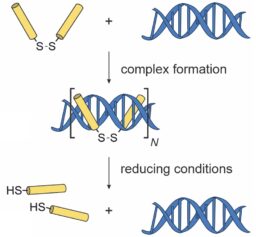
Environment-Responsive Peptide Dimers Bind and Stabilize Double-Stranded RNA
NM McLoughlin, MA Albers, E Collado Camps, J Paulus, YA Ran, S Neubacher, S Hennig, R Brock, TN Grossmann Angew. Chem. Int. Ed. e202308028.
Great collaboration with the Brock lab at Radboud University!

We enjoyed the AIMMS Festival in Egmond aan Zee. Great contributions by our team! Congratulations to Felix for receiving a poster prize!
Read more about Felix’ project here: FM Paulussen et al. J. Am. Chem. Soc. DOI: 10.1021/jacs.2c06304

Jannik joins us from Norbert Sewald’s group and we’re happy about the additional peptide synthesis expertise!
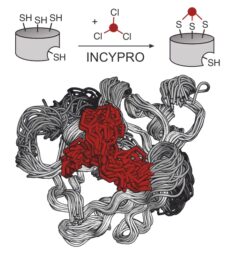
Bicyclic Engineered Sortase A Performs Transpeptidation under Denaturing Conditions
S Kiehstaller, GH Hutchins, A Amore, A Gerber, M Ibrahim, S Hennig, S Neubacher, TN Grossmann, Bioconj. Chem. DOI: 10.1021/acs.bioconjchem.3c00151

The KWF funded project ‘WINCE: Wnt inhibitors to treat colorectal cancer’ (joint with the Vermeulen lab) has started. It’s based on our Wnt inhibitors:
M Wendt et al. Angew. Chem. DOI: 10.1002/anie.202102082
L Dietrich et al. Cell Chem. Biol. DOI: 10.1016/j.chembiol.2017.06.013
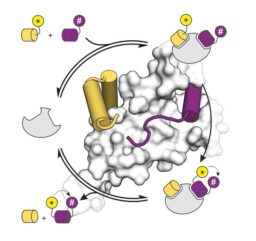
Non-enzymatic protein templates amide bond formation and provides catalytic turnover
N Brauckhoff, L Fang, A Haim, TN Grossmann, ChemComm, DOI: 10.1039/d3cc00514c

The ERC funded project ‘grabRNA: Smart RNA delivery for therapy and diagnostic’ has started. It’s based on our peptidic RNA binders:
A Kuepper et al. Nucleic Acids Res. DOI: 10.1093/nar/gkab1149
NM McLoughlin et al. Chem. Eur. J. DOI: 10.1002/chem.202101103

Welcome Eni Rile, Marvin Albers and Anissa Haim! Great to have you onboard!

Peptide-based covalent inhibitors of protein-protein interactions
FM Paulussen, TN Grossmann, J. Pept. Sci. DOI: 10.1002/psc.3457
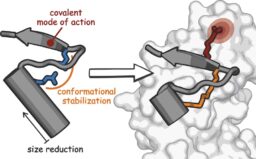
Covalent Proteomimetic Inhibitor of the Bacterial FtsQB Divisome Complex
FM Paulussen et al. J. Am. Chem. Soc. DOI: 10.1021/jacs.2c06304
Congratulations to the entire team at the Vrije Universiteit Amsterdam and Universiteit van Amsterdam! Great collaboration with the groups of Joen Luirink, Tanneke den Blaauwen, Peter van Ulsen, and Daan Geerke.